FDA Approves Zepbound for Apnea (Obstructive Sleep)
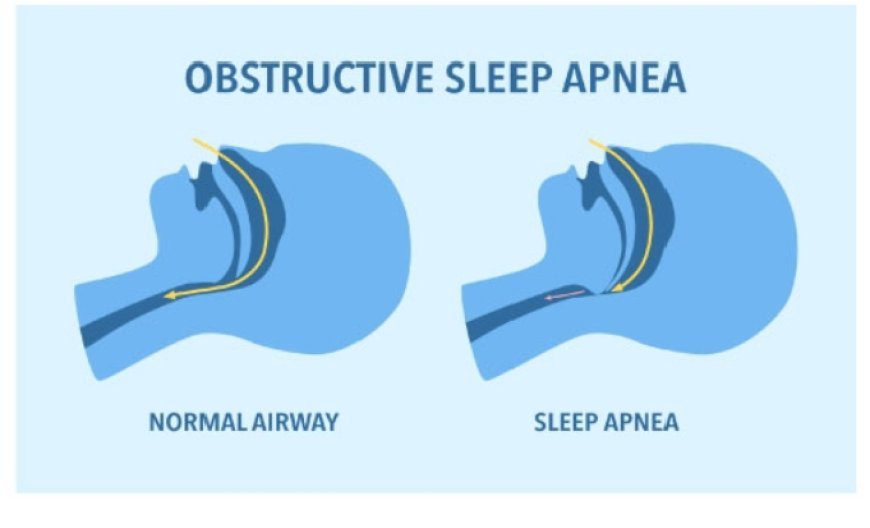
FDA Approves Zepbound for Apnea (Obstructive Sleep) FDA Approves Zepbound for Apnea (Obstructive Sleep) FDA has approved the first prescription drug, Zepbound, for treating moderate to severe obstructive sleep (OSA) Apena in adults with obesity. Studies have shown that when combined with a low-calorie diet and increased exercise, Zepbound improves OSA symptoms by reducing body weight, particularly in those with weight-related health issues. OSA is a breathing disorder where the upper airway becomes partially or fully blocked during sleep, causing shallow breathing or brief pauses in breathing (apnea), often followed by gasping, snorting, or waking suddenly. OSA can reduce oxygen flow, disrupt heart rhythms, and harm overall health. Common signs such as snoring, tiredness, daytime sleepiness, and disrupted sleep often go unnoticed. OSA is more common in people with obesity, as excess neck weight can press down on the airway. Symptoms of Apena. 1)Frequent lou d snoring 2)Pause in breathing 3) Difficult with memory with memory and concentration 4)Frequent uranate at night 5)Dry Mouth 6)Erectile dyfunction. CHISHTY AZHARUDDIN. INVESTIGATIVE JOURNALIST IHRC NEWS INDIA .
1. FDA Approves Zepbound for Apnea (Obstructive Sleep)

What's Your Reaction?